configuration of sulphur|Sulfur Electron Configuration (S) with Orbital Diagram : iloilo How to Write the Electron Configuration for Neon. Neon is the tenth element with a . The cheapest way to get from Dumaguete to Butuan is to ferry and bus via Cebu City which costs $30 - $280 and takes 18h 54m. More details. . Butuan, officially the City of Butuan (Cebuano: Dakbayan sa Butuan; Butuanon: Dakbayan hong Butuan; Filipino: Lungsod ng Butuan), is a 1st class highly urbanized city in the region of Caraga, .
configuration of sulphur,In order to write the Sulfur electron configuration we first need to know the number of electrons for the S atom (there are 16 electrons). When we write the configuration we'll put all 16 electrons in orbitals around the nucleus of the Sulfur atom.How to Write the Electron Configuration for Neon. Neon is the tenth element with a .
When we write the configuration we'll put all 18 electrons in orbitals around the .In order to write the Calcium electron configuration we first need to know the .
The next six electrons will go in the 2p orbital. The p orbital can hold up to six .The next six electrons will go in the 2p orbital. The p orbital can hold up to six .Therefore the N electron configuration will be 1s 2 2s 2 2p 3. Video: Nitrogen . Ground State Electron Configuration of Sulfur. when we the electron configuration of Sulfur the first two electrons go in the 1s orbital. As 1s only hold two electrons and the next two electrons for . Sulfur Electron Configuration - YouTube. Wayne Breslyn. 751K subscribers. Subscribed. 441. 98K views 10 years ago. A step-by-step description of . The sulfur electron configuration, represented as [ Ne] 3s 2 3p 4 or 1s 2 2s 2 2p 6 3s 2 3p 4, illustrates the arrangement of electrons within the atom. This configuration can be determined through various .We will now construct the ground-state electron configuration and orbital diagram for a selection of atoms in the first and second periods of the periodic table. Orbital diagrams .Sulfur was mined near Mount Etna in Sicily and used for bleaching cloth and preserving wine, both of which involved burning it to form sulfur dioxide, and allowing this to be . To write the orbital diagram for the Sulfur atom (S) first we need to write the electron configuration for just S. To do that we need to find the number of electrons for the S atom (there are.
The electron configuration for sulfur is 1s 2 2s 2 2p 6 3 s 2 3p 4 and can be represented using the orbital diagram below. The electron configuration of sulfur is 1s2 2s2 2p6 3s2 3p4, if the electron arrangement is through orbitals. Sulfur is the 16th element in the periodic table. Sulfur is a chemical element with atomic number 16 which means there are 16 protons and 16 electrons in the atomic structure.The chemical symbol for Sulfur is S. Electron Configuration and Oxidation States of Sulfur. Electron configuration of Sulfur is [Ne] 3s2 3p4. Possible oxidation states are +4,6/-2. Electron ConfigurationElectronic configuration for Sulfur (S) The atomic number of Sulfur is 16. Sulfur belongs to group 16 of the Modern periodic table. In the periodic table, Sulfur is placed in the third period. For the electronic configuration of an element there are three important rules which must be followed: Aufbau Principle Pauli-exclusion Principle Hund's . Therefore, the electronic configuration of sulphur is: \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^4}\] Note: Make sure to remember that the energy of 4s orbital is less than the energy of 3d orbital. There are more exceptions in the energy levels of orbitals which can be checked using the Aufbau Rule.

Consider sulfur's electron configuration, which was determined in the previous section and is replicated below. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 4. Recall that the energy levels in an electron configuration are the leading red numbers that denote the start of a new energy level / orbital combination.Sulfur Electron Configuration (S) with Orbital Diagram Consider sulfur's electron configuration, which was determined in the previous section and is replicated below. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 4. Recall that the energy levels in an electron configuration are the leading red numbers that denote the start of a new energy level / orbital combination.

Sulfur is the SIXTH element in the THIRD row of the periodic table.So it is the same as Neon, but with a full 3s2 subshell and four electrons in its 3p.When .configuration of sulphur Sulfur is the SIXTH element in the THIRD row of the periodic table.So it is the same as Neon, but with a full 3s2 subshell and four electrons in its 3p.When . Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.The chemical symbol for Hydrogen is H. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the . The sulfur electron configuration can also be written using ochemberlin terms, which are another way to denote electron orbital levels. In this notation, the sulfur electron configuration would be written as 4s2 4p4. This means that there are two electrons in the 4s orbital and four electrons in the 4p orbitals.
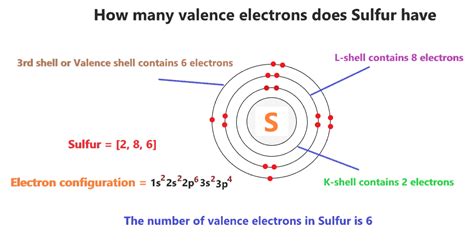
Here is the way you can follow to write the electron configuration og sulphur(S) in just 5 steps. Step-1: To do electron configuration of sulphur, we have to know the atomic number of sulpur(S). The atomic number of sulphur is 16.So sulpur has 16 electrons and 16 protons. Electron configuration .Thus, electronic configuration of sulphur K (2), L (8), M (6) Number of orbit in sulphur = 3. Was this answer helpful? 3. Similar Questions. Q1. Equilibrium constants K 1 and K 2 for the following equilibria are.configuration of sulphur Sulfur Electron Configuration (S) with Orbital DiagramThus, electronic configuration of sulphur K (2), L (8), M (6) Number of orbit in sulphur = 3 Solve any question of Classification Of Elements And Periodicity In Properties with:- Sulfur Electron Configuration: Sulphur or sulfur is a chemical element.It has a chemical symbol S. The atomic number of Sulfur is 16. It is multivalent, abundant, and nonmetallic. Under normal .The electron configuration of an oxygen atom [He] 2s 2 2p 4 suggests that neutral oxygen atoms can achieve an octet of valence electrons by sharing two pairs of electrons to form an O=O double bond, as shown in .
Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain of electrons in their subsequent orbitals. Many of the physical and chemical properties of elements can be correlated . A step-by-step description of how to write the electron configuration for Sulfur (S). In order to write the S electron configuration we first need to know t.In cosmic abundance, sulfur ranks ninth among the elements, accounting for only one atom of every 20,000–30,000. Sulfur occurs in the uncombined state as well as in combination with other elements in rocks and minerals that are widely distributed, although it is classified among the minor constituents of Earth’s crust, in which its proportion is estimated to be .
The first three quantum numbers of an electron are n=1, l=0, m l =0. Only two electrons can correspond to these, which would be either m s = -1/2 or m s = +1/2. As we already know from our studies of quantum numbers and electron orbitals, we can conclude that these four quantum numbers refer to the 1s subshell.
To write the orbital diagram for the Sulfur atom (S) first we need to write the electron configuration for just S. To do that we need to find the number of e.
The general electronic configuration for sulphur by the Bohr’s atomic theory can be stated as 2, 8, 6 simply. Do note that the electronic configuration can also be stated including the noble gas element as; $\left[ Ne \right]3{{s}^{2}}3{{p}^{4}}$ . Recently Updated Pages.
configuration of sulphur|Sulfur Electron Configuration (S) with Orbital Diagram
PH0 · Sulphur Electron Configuration: How Electrons Are Distributed
PH1 · Sulfur electron configuration
PH2 · Sulfur Electron Configuration (S) with Orbital Diagram
PH3 · Sulfur Electron Configuration
PH4 · Sulfur
PH5 · How to Write the Orbital Diagram for Sulfur (S)
PH6 · Electron Configuration for Sulfur (S)
PH7 · Complete Electron Configuration for Sulfur (S, S2
PH8 · 6.4 Electronic Structure of Atoms (Electron Configurations)
PH9 · 1.4: Electron Configurations and Electronic Orbital Diagrams